Search
Research
The impact of obesity on influenza Vaccine immunogenicity - A systematic reviewInfluenza vaccines are important for reducing the burden of influenza, particularly for populations at risk of more severe infections. Obesity is associated with increased influenza severity and therefore individuals with obesity are often specifically recommended for annual influenza vaccination. Obesity is also associated with an altered inflammatory profile, which may influence vaccine responses. This systematic review aimed to evaluate the evidence for any association between obesity and influenza vaccine immunogenicity.
Research
Predicting regional and temporal incidence of RSV and influenza hospitalizations in a birth cohort of young Australian childrenWestern Australia experiences multiple climatic zones, influencing the epidemiology of respiratory viruses. We aimed to estimate the true incidence of respiratory syncytial virus and influenza hospitalizations across these different climatic regions using predictive modelling.
Research
Characterising the SARS-CoV-2 nucleocapsid (N) protein antibody responseSARS-CoV-2 nucleocapsid (N) protein antibodies can be used to identify the serological response to natural infection in those who have previously received a COVID-19 spike-based vaccine. Anti-N antibody responses can also be induced by inactivated whole SARS-CoV-2 virus vaccines, such as CoronaVac. We aimed to characterise antibody responses to the N protein following COVID-19 and following vaccination with CoronaVac.
Research
Effectiveness of nirsevimab in preventing RSV-hospitalisation among young children in Western Australia 2024Respiratory Syncytial Virus (RSV) causes a significant burden of illness for children under 2 years of age. Nirsevimab, a long-acting monoclonal antibody, was registered for RSV prevention in Australia in 2023. In April 2024, Western Australia (WA) launched the country's first state-wide nirsevimab program for all infants and high-risk children entering their second RSV season.
Research
ATOMIC Ears: A Phase IIB randomised controlled trial to assess safety, tolerability and acceptability of a 5-day Dornase alfa treatment as an adjunct therapy to ventilation tube insertion for otitis media in childrenChris Jennifer Lea-Ann Peter Ruth Brennan-Jones Kent Kirkham Richmond Thornton PhD RN PhD MBBS MRCP(UK) FRACP PhD Head, Ear and Hearing Health
Research
COVALIA (COVid vaccine trial for austrALIA): A phase I, double-blind, dose-ranging, randomised, placebo-controlled trial to study the safety and immunogenicity of a DNA-based vaccine against COVID-19 (COVIGEN) in healthy participants aged 18 to 75 years oPeter Richmond MBBS MRCP(UK) FRACP Head, Vaccine Trials Group Head, Vaccine Trials Group Professor Peter Richmond is Head of the Vaccine Trials Group
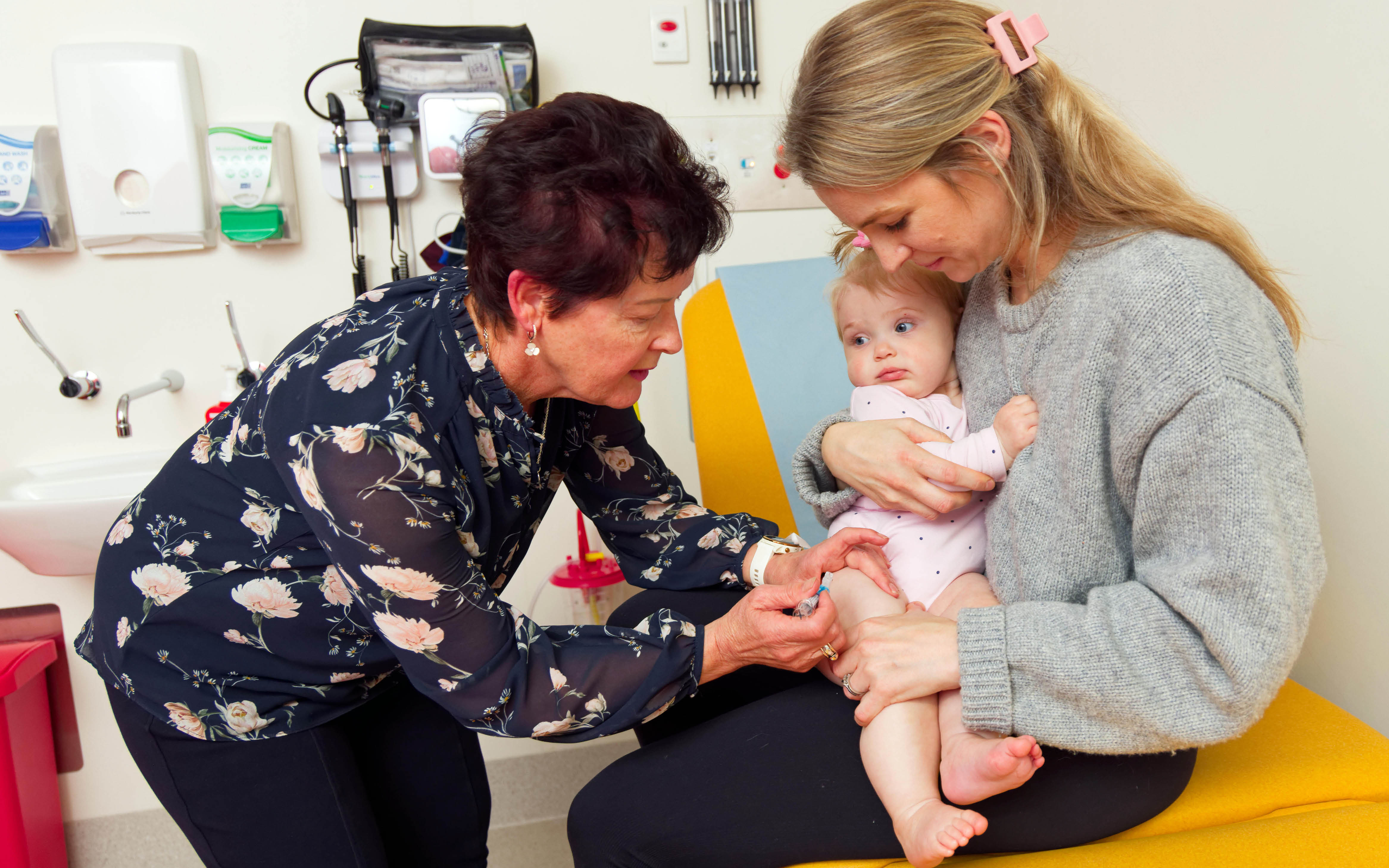
The mission of the Vaccine Trials Group is to improve the health of the community through immunisation and the prevention of infectious diseases.
Research
A phase 3, multicenter, randomized, double-blind, active comparator-controlled study to evaluate the safety and tolerability of V114 in healthy infants (PNEULINK)Jennifer Peter Kent Richmond RN MBBS MRCP(UK) FRACP Clinical Research Manager Head, Vaccine Trials Group Jennifer.Kent@thekids.org.au Clinical
Research
Human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for the prevention of cervical cancer and HPV-related diseasesHPV-16/18 AS04-adjuvanted vaccine reduces HPV-16/18 infection and associated cervical endpoints in women regardless of age, location, or sexual experience
Research
The effectiveness of influenza vaccination in preventing hospitalisation in children in Western AustraliaThis study aimed to determine the vaccine effectiveness of the southern hemisphere trivalent inactivated influenza vaccine (TIV) in preventing...
