Search

News & Events
Latest Deborah Lehmann Research Award recipient tackles malaria in MadangPapua New Guinean researcher Dr Lincoln Timinao has been awarded the 2025 Deborah Lehmann Research Award (DLRA) for his work aimed at investigating the burden of malaria in young children.
News & Events
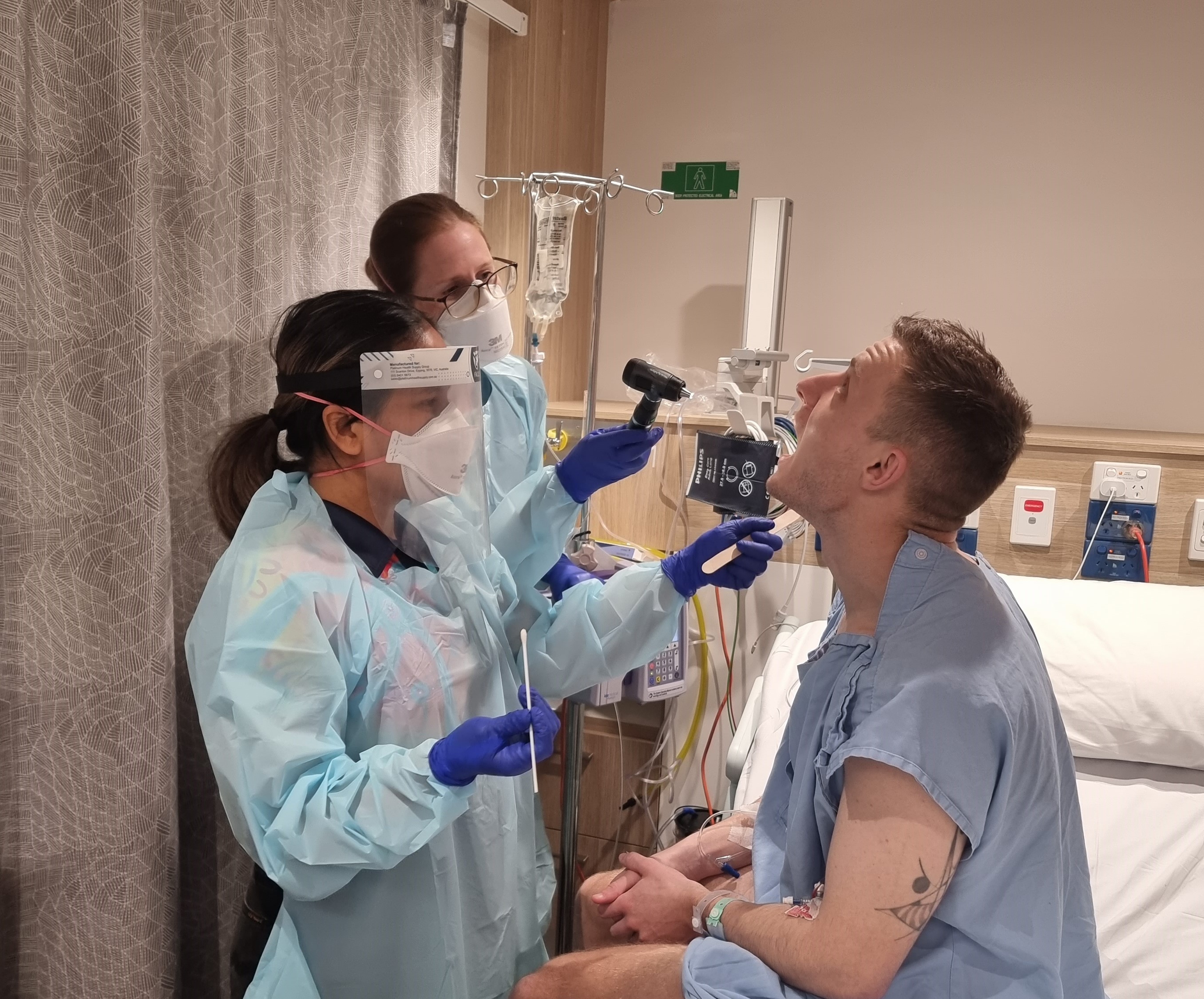
Study which deliberately infected participants leads to penicillin breakthroughA unique study purposely giving participants Streptococcus pyogenes (Strep A) to learn how much penicillin it takes to prevent infection has found the amount needed is much lower than previously thought – a discovery that will transform thinking on treatment for people living with rheumatic heart disease (RHD).

News & Events
New tool guides families on RSV immunisationResearchers from the Wesfarmers Centre of Vaccines and Infectious Diseases, based at The Kids Research Institute Australia, have launched an online guidance tool designed to help families and health-care providers in WA learn the best way to protect babies and young children against life-threatening respiratory syncytial virus (RSV).

News & Events
WA Health funding supports development of rapid test for antibiotic-resistant skin infections in Aboriginal childrenA rapid test to detect antibiotic-resistant skin infections in Aboriginal children could be a step closer, thanks to support from the FHRIF.

News & Events
World-first trial to seek child-specific treatments for dangerous bloodstream infectionsThe Kids Research Institute Australia, Perth Children’s Hospital (PCH) and the Peter Doherty Institute for Infection and Immunity (Doherty Institute) will spearhead the paediatric arm of a world-first global platform trial designed to uncover treatments for Staphylococcus aureus bloodstream infection.

News & Events
Flu study gives vital early protection to bubs this winterThe FluBub Study will investigate whether giving the flu vaccine much earlier than the six months currently recommended by the National Immunisation Program will protect babies at the greatest risk of a severe influenza infection when they are most vulnerable.

News & Events
The Kids Research Institute Australia launches Covid-19 booster research to inform Australia’s vaccine policyOptimising our national Covid-19 vaccine program could be one step closer thanks to new research now underway at The Kids Research Institute Australia investigating the most effective, long-term strategies for booster vaccinations.

News & Events
New Aboriginal Cultural Guidance Advisor appointedThe Wesfarmers Centre of Vaccines and Infectious Diseases has appointed Mrs Valerie Swift to a newly created Aboriginal Cultural Guidance Advisor position.

News & Events
Round one done, eight to go for the SToP TrialSix weeks, nine community visits and 380 kids – it’s a wrap for round one of the StoP Trial!

News & Events
Early ear infections linked to higher risk of future problems: studyResearchers have found kids who experience repeat ear infections in infancy have a much higher risk of ongoing problems with ear infections in later childhood
